Osteoporosis and the Centrality of Bone Mineral Density Measurement
1. Samatbek Turdaliev
2. Abhijeet Singh Choudary
Mohini Choudhary
(1. Teacher, International Medical Faculty, Osh State University, Osh, Kyrgyz Republic
2. Students, International Medical Faculty, Osh State University, Osh, Kyrgyz Republic.)
Abstract
Background: Osteoporosis is a systemic skeletal disease defined by compromised bone strength and microarchitectural degradation, leading to an increased risk of fragility fractures. The accelerating global prevalence necessitates rigorous methods for early diagnosis and intervention. The concept of Bone Mineral Density (BMD) serves as the indispensable clinical benchmark for assessing bone mass.
Methods: This review synthesizes the cellular mechanisms governing BMD homeostasis, evaluates the diagnostic utility of BMD measurement, and critiques the integration of BMD data with clinical risk assessment tools and therapeutic strategies.
Results: The central defect in osteoporosis is an imbalance in the Bone Remodeling Unit (BMU), characterized by bone resorption (osteoclasts) exceeding bone formation (osteoblasts), largely mediated by the RANK/RANKL/OPG system. The gold standard for diagnosis is Dual-energy X-ray Absorptiometry (DXA), which quantifies BMD and establishes osteoporosis via a T-score of -2.5 or less. While BMD is the strongest predictor of fracture risk, its limitation is the failure to capture bone microarchitecture (quality).
Discussion: Clinical practice has evolved to integrate BMD with clinical factors using tools like FRAX to accurately calculate the 10-year fracture probability, especially in the osteopenic range. Pharmacological agents act either as anti-resorptives (e.g., bisphosphonates, Denosumab) to increase BMD by suppressing osteoclasts, or as potent anabolics (e.g., Teriparatide) to stimulate new bone formation. Future diagnostics must aim to quantify bone quality to fully capture fracture risk.
Keywords: Osteoporosis; Bone Mineral Density (BMD); Dual-energy X-ray Absorptiometry (DXA); T-score; Bone Remodeling; FRAX; Anti-resorptive; Anabolic.
Introduction
i. Contextualizing Osteoporosis as a Global Health Crisis
Osteoporosis, literally translating to "porous bone," is a systemic skeletal disease characterized by compromised bone strength predisposing an individual to an increased risk of fracture. This deterioration stems from reduced bone mass and microarchitectural degradation of bone tissue. While often viewed as a natural consequence of aging, particularly post-menopause, its ramifications extend far beyond simple fragility, contributing significantly to chronic pain, physical disability, loss of independence, and substantial healthcare expenditure worldwide. The most devastating consequence of osteoporosis is the fragility fracture, with vertebral, hip, and distal forearm fractures being the most common. The burden of osteoporotic hip fractures, in particular, carries a high rate of mortality within one year of the event (ranging from 20% to 30%) and leaves a majority of survivors requiring long-term care. Given the accelerating aging of the global population, the public health crisis posed by osteoporosis is expanding exponentially, demanding rigorous approaches to early diagnosis, risk stratification, and proactive intervention.
ii. The Centrality of Bone Mineral Density (BMD)
The operational definition of osteoporosis and the primary mechanism for its diagnosis is the measurement of Bone Mineral Density (BMD). BMD is a quantitative measure of the amount of mineral (primarily calcium phosphate) contained per unit volume or area of bone tissue, typically expressed in grams per square centimeter (g/cm2). The gold standard technique for measuring BMD is Dual-energy X-ray Absorptiometry (DXA), which provides precise and accurate measurements at clinically relevant sites such as the lumbar spine, proximal femur, and occasionally the forearm. The WHO definition of osteoporosis is explicitly linked to a T-score of -2.5 or less at the femoral neck, spine, or forearm in postmenopausal women and men aged 50 years and older. This T-score represents the number of standard deviations the patient's BMD is below the mean peak bone mass of a young, healthy adult of the same sex. While BMD accounts for approximately 70% of bone strength and is the most powerful predictor of future fracture risk, it is critical to recognize that bone strength is a composite attribute, also profoundly influenced by bone microarchitecture, bone turnover rate, and the accumulation of microdamage. Nonetheless, BMD remains the indispensable surrogate marker for clinical practice, directing all screening and therapeutic decisions.
iii. Scope and Rationale of the Review
This comprehensive academic review is designed to provide a deep analysis of the pathophysiology of osteoporosis, focusing specifically on the central role of Bone Mineral Density in its diagnosis, risk assessment, and management. We will explore the intricate cellular mechanisms (osteoclast/osteoblast interplay) that govern BMD homeostasis and detail the factors (genetic, hormonal, environmental) that contribute to its decline. Furthermore, the review will critically evaluate the utility and limitations of the DXA-derived T-score and discuss its integration with fracture risk assessment tools, such as FRAX. Finally, we will synthesize the current evidence regarding therapeutic interventions, classifying them based on their direct mechanism of action on BMD maintenance or augmentation. This holistic approach aims to provide a robust framework for understanding and combating this pervasive skeletal disorder.
Methods
i. Study Design and Scope
This academic review employs a comprehensive, non-systematic methodology, integrating evidence from basic science research, large-scale epidemiological studies, randomized controlled trials (RCTs), and established clinical practice guidelines. The primary objective was to investigate the biological underpinnings of Bone Mineral Density regulation and its application as the cornerstone diagnostic and predictive tool for Osteoporosis. The scope encompasses primary (postmenopausal and senile) osteoporosis and essential aspects of secondary osteoporosis related to underlying medical conditions or medications.
ii. Literature Search Strategy
A multi-database literature search was executed using electronic resources, including PubMed/MEDLINE, Scopus, and the Cochrane Library. The search strategy employed both controlled vocabulary (MeSH terms) and keywords, utilized in various combinations with Boolean operators (AND, OR). Key search terms included: "Osteoporosis Pathophysiology," "Bone Mineral Density (BMD)," "DXA," "T-score," "Osteoblast," "Osteoclast," "Bone Remodeling," "Fracture Risk Assessment," and "Anti-resorptive Therapy." The search was prioritized for publications released within the last two decades (2005 to 2024) to ensure the inclusion of contemporary understanding of molecular targets and updated guidelines on pharmacological therapy, although seminal works establishing the DXA T-score criteria were included for foundational context.
iii. Inclusion and Exclusion Criteria
Articles were selected for inclusion if they: (a) provided original data on the cellular or molecular mechanisms regulating bone turnover; (b) evaluated the predictive power of DXA-derived BMD measurements for incident fragility fractures; (c) assessed the efficacy of anti-osteoporotic pharmacological agents on BMD change and fracture reduction; or (d) discussed clinical guidelines regarding screening and risk stratification. Exclusion criteria included: articles focusing exclusively on non-BMD related bone diseases (e.g., osteomalacia, Paget's disease), studies limited to pediatric populations without relevance to peak bone mass acquisition, and non-peer-reviewed or low-quality grey literature. Data quality was assessed based on the study design, rigor, and the size of the patient cohorts examined.
iv. Data Synthesis and Analysis
The extracted scientific and clinical data were systematically organized and analyzed thematically, focusing on four pillars: Cellular and Molecular Homeostasis, Diagnostic Framework (BMD/T-score), Epidemiology and Risk Factors, and Therapeutic Mechanisms. Synthesis focused on integrating the basic science of bone remodeling with the clinical application of DXA technology. Specifically, the analysis aimed to articulate the limitations of BMD as a sole predictor of fracture risk and to highlight the benefit of combining it with clinical risk factors (e.g., via FRAX) to enhance predictive accuracy and guide intervention thresholds. The final synthesis critiqued the evolving treatment landscape based on its demonstrated effect on augmenting BMD and reducing fracture incidence.
Results
i. Cellular and Molecular Basis of BMD Homeostasis
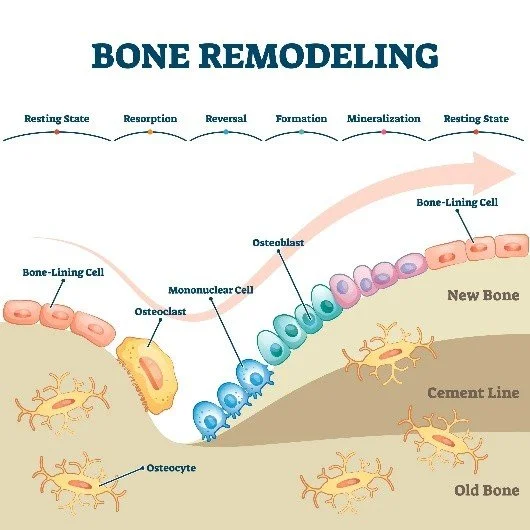
Bone is a dynamic tissue constantly undergoing a tightly regulated process known as bone remodeling, which is essential for maintaining mechanical strength, repairing microdamage, and mineral homeostasis. This process is carried out by the Basic Multicellular Unit (BMU), involving a coordinated sequence of resorption by osteoclasts and formation by osteoblasts. The balance between the activity of these two cell types is the primary determinant of long-term Bone Mineral Density. Osteoporosis arises when this balance is tipped, such that bone resorption exceeds bone formation.
The critical molecular axis governing this imbalance is the RANK/RANKL/OPG system. Receptor Activator of Nuclear Factor kB Ligand (RANKL), expressed on osteoblasts and stromal cells, binds to its receptor, RANK, on the surface of osteoclast precursors, leading to their differentiation, activation, and survival, thus promoting bone resorption. Osteoprotegerin (OPG), a soluble decoy receptor also produced by osteoblasts, acts as a brake on this process by competitively binding to RANKL, preventing it from activating RANK. A reduction in the OPG:RANKL ratio—often seen post-menopause due to oestrogen deficiency—drives rampant osteoclast activity, accelerates bone loss, and leads to the decline in BMD characteristic of primary osteoporosis. Furthermore, systemic factors such as parathyroid hormone (PTH), Vitamin D metabolites, and various cytokines modulate this BMU activity, influencing the overall rate of bone turnover and the trajectory of BMD loss over an individual's lifetime.
ii. Quantification of BMD and the T-Score Framework
The diagnosis of osteoporosis hinges on the quantification of Bone Mineral Density using Dual-energy X-ray Absorptiometry (DXA). DXA provides areal density (g/cm2) measurements, primarily at the lumbar spine, total hip, and femoral neck, as these sites are predictive of the most devastating fractures. To normalize for sex and age, DXA results are expressed as two key scores: The T-score and the Z-score.
The T-score is the cornerstone of clinical diagnosis, defined as the number of standard deviations the patient's BMD falls below the mean peak BMD of a young, healthy, sex-matched reference population. The World Health Organization (WHO) established diagnostic criteria based on the T-score for postmenopausal women and men aged ≥ 50 years:
· Normal: T-score ≥ -1.0
· Osteopenia (Low Bone Mass): T-score between -1.0 and -2.5
· Osteoporosis: T-score ≤ -2.5
The Z-score represents the number of standard deviations the patient's BMD is below the mean BMD of an age-matched and sex-matched population. This score is primarily used for diagnosing secondary osteoporosis in premenopausal women, men under age 50, and children, where a Z-score -2.0 is considered "below the expected range for age." While the T-score is highly reproducible and demonstrates a robust inverse correlation with fracture risk, its limitation lies in the fact that it only reflects density, not bone quality (microarchitecture), which is critical in determining the full biomechanical strength of the skeleton.
iii. Epidemiology and Risk Factors for BMD Decline
The lifetime risk for an osteoporotic fracture in adults aged 50 years and older is approximately $50\%$ for women and 20% for men, underscoring the high prevalence of the underlying loss of BMD. The decline in BMD is a multifactorial process. Unmodifiable Risk Factors include female sex, advanced age, Caucasian or Asian ethnicity, a family history of osteoporosis, and small body frame. Modifiable Risk Factors are critical targets for intervention and include inadequate dietary calcium and Vitamin D intake, sedentary lifestyle, excessive alcohol consumption, smoking, and low body weight (BMI < 18.5 kg/m2). Furthermore, a substantial number of cases are due to Secondary Osteoporosis, driven by chronic diseases such as rheumatoid arthritis, hyperparathyroidism, hyperthyroidism, chronic kidney disease, and, most commonly, the long-term use of glucocorticoids, which directly inhibit osteoblast function and promote osteoclast activity, leading to rapid and profound BMD loss. The identification of these risk factors necessitates a comprehensive clinical history alongside BMD measurement to accurately predict fracture probability.
Discussion
The central role of Bone Mineral Density in the diagnosis of osteoporosis is indisputable, yet relying on the T-score alone fails to capture the full spectrum of fracture risk, especially in the osteopenic range. A holistic approach integrating BMD with clinical risk factors has become the current standard of care, dictating the necessity and timing of pharmacological intervention.
i. Beyond the T-Score: Integrating BMD with Clinical Risk via FRAX
The fracture risk assessment tool FRAX (Fracture Risk Assessment Tool) was developed by the WHO to integrate BMD data with established clinical risk factors, thus refining the prediction of fracture probability. FRAX calculates the 10-year probability of a major osteoporotic fracture (hip, clinical spine, forearm, or proximal humerus) and, separately, the 10-year probability of a hip fracture. Clinical risk factors incorporated into the FRAX model include age, sex, body mass index, prior fragility fracture history (the single strongest predictor), parental history of hip fracture, current smoking, glucocorticoid use, excessive alcohol intake, rheumatoid arthritis, and other causes of secondary osteoporosis. This synergistic approach allows for the identification and treatment of patients who are technically categorized as osteopenic (T-score > -2.5) but possess high clinical risk factors that place their overall fracture risk equivalent to or higher than patients with frank osteoporosis. For example, a low BMD may be treated aggressively if the patient has had a previous fragility fracture or is receiving high-dose glucocorticoid therapy, demonstrating the clinical utility of moving beyond a simple threshold diagnosis.
ii. Pharmacological Interventions and Their Mechanism on BMD
Pharmacological management aims to shift the bone remodeling balance back in favour of formation or reduce excessive resorption, thereby increasing BMD and reducing fracture incidence. These therapies can be broadly classified by their primary mechanism of action:
Anti-resorptive Agents: These drugs primarily inhibit osteoclast function, slowing down the rate of bone resorption and allowing for a gradual net gain in BMD over time. The most widely used class is the bisphosphonates (e.g., alendronate, zoledronic acid), which are incorporated into the bone matrix and, when ingested by osteoclasts, induce apoptosis and impair their function. Other highly effective anti-resorptives include Denosumab, a fully human monoclonal antibody that directly targets and inhibits RANKL, thereby preventing osteoclast formation and activation. By blocking the fundamental resorption signal, Denosumab achieves potent, rapid suppression of bone turnover and substantial increases in BMD.
Anabolic Agents: These agents stimulate the activity of osteoblasts, promoting new bone formation, leading to a more rapid and robust increase in BMD compared to anti-resorptives. Teriparatide and Abaloparatide are synthetic analogues of Parathyroid Hormone (PTH) that, when administered intermittently, exert a net anabolic effect. They are typically reserved for patients with severe osteoporosis or those who have failed previous anti-resorptive therapy. A newer class, Romosozumab, provides a dual action by simultaneously inhibiting Sclerostin (a negative regulator of bone formation) and modestly enhancing bone resorption, offering a powerful, time-limited therapeutic window. These anabolic agents are critical for patients who have suffered a fracture despite high BMD.
iii. Challenges in Measurement and Therapeutic Monitoring
Despite its strengths, DXA measurement is subject to several limitations. First, in the lumbar spine, degenerative changes (osteoarthritis, vertebral compression fractures) can artificially elevate the BMD reading, potentially masking true osteoporosis. Second, DXA provides only a two-dimensional areal measurement, neglecting bone geometry and cortical thickness, which are independent determinants of strength. Third, the long-term effectiveness of osteoporosis therapy is largely monitored by tracking changes in BMD. However, achieving a measured BMD increase does not always directly correlate with a proportional reduction in fracture risk, especially in older patients whose bones are severely compromised microarchitecturally. The use of Bone Turnover Markers (BTMs)—such as C-telopeptides (CTX) for resorption and Procollagen type I N-terminal Propeptide (P1NP) for formation—is increasingly used to monitor patient adherence and drug effectiveness more rapidly than sequential BMD measurements.
iv. Conclusion and Future Directions
Osteoporosis remains a pervasive and costly skeletal disease, demanding continuous commitment to early detection and aggressive management. The concept of Bone Mineral Density, defined by the T-score and quantified by DXA, serves as the critical entry point for diagnosis and risk stratification. However, clinical practice has evolved beyond the simple T-score threshold to incorporate the FRAX tool, allowing for a more accurate assessment of individual patient fracture probability based on the synergy between density and clinical risk factors. Future advances in the field will focus on high-resolution imaging techniques, such as High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT), which allow for true three-dimensional analysis of bone microarchitecture, providing a more complete picture of bone strength independent of density alone. The continued development of new anabolic and anti-resorptive therapies promises to further reduce the public health burden of fragility fractures, moving toward a future where fracture prevention is both precise and widely accessible.
References
1. Kanis JA, Oden A, Johnell O, et al. The worldwide problem of osteoporosis and fracture: background and analysis. Osteoporos Int. 2011;22(Suppl 3):31-37.
2. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis: 2020 update. Endocr Pract. 2020;26(Suppl 1):1-46.
3. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. Geneva: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276-1287.
5. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504-1508.
6. Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104(5):1521-1543.
7. Compston J, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364-376.
8. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
9. Cummings SR, Black DM. Bone mass measurements and the diagnosis of osteoporosis. JAMA. 1995;273(1):76-77.